- Nitrogen Atomic Weight Of Gas
- Nitrogen Atomic Mass
- Atomic No Of Nitrogen Symbol
- Atomic No Of Nitrogen Cycle
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 14N | 14.003 074 004(2) | [0.995 78, 0.996 63] |
| 15N | 15.000 108 899(4) | [0.003 37, 0.004 22] |

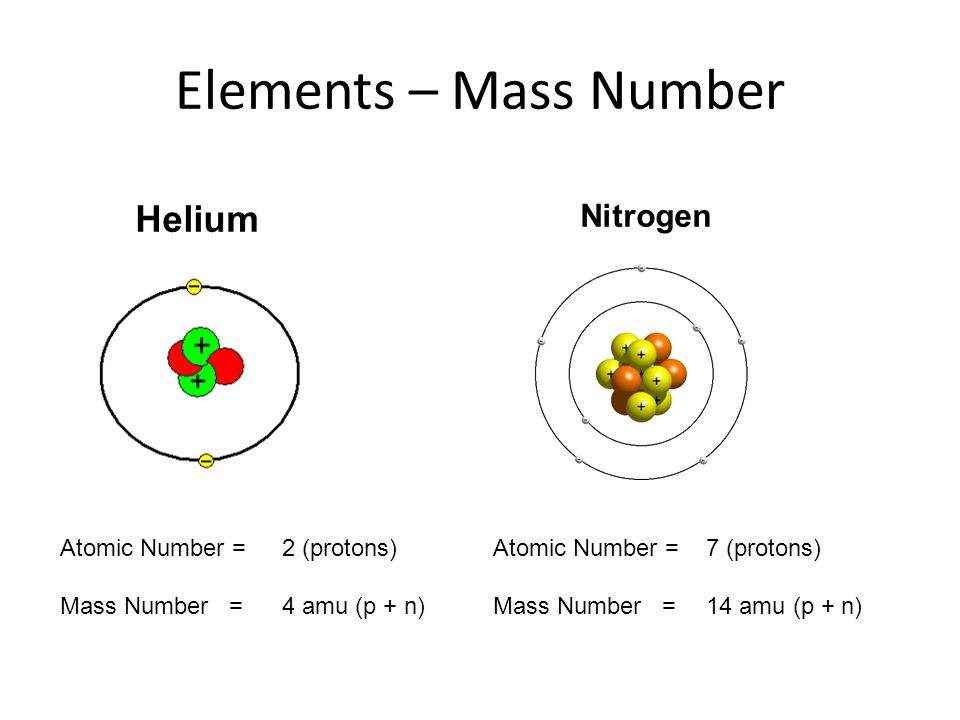
Name: Nitrogen Symbol: N Atomic Number: 7 Atomic Mass: 14.00674 amu Melting Point:-209.9 °C (63.250008 K, -345.81998 °F) Boiling Point:-195.8 °C (77.35 K, -320.44 °F) Number of Protons/Electrons: 7 Number of Neutrons: 7 Classification: Non-metal Crystal Structure: Hexagonal Density @ 293 K: 1.2506 g/cm 3 Color: colorless Atomic Structure. Fatal Accident at Valero RefineryDelaware City, DE, November 5, 2005Two contract employees were overcome and fatally injured by nitrogen as they performed ma.
The primary reference material for the relative abundance measurements of nitrogen isotopes is atmosphericN2, which is homogeneous with respect to analytical uncertainties and is assigned aδ15Nair value of 0 ‰. Relative isotope-ratio measurements of nitrogen commonly have uncertainties of the order of 0.1 ‰,which is significantly smaller than the reported uncertainty of the calibrated 'best measurement'(1.1 ‰). Variations in the isotopic composition of nitrogen in chemical reagents and natural terrestrialsystems are known to exceed 200 ‰, which is much larger than the uncertainty due to either relative or'absolute' isotope-ratio measurements. Therefore, the accuracy and precision of the standard atomicweight of nitrogen are limited almost entirely by real variations, hence the annotation 'r'.
Measurable variations in the isotope abundances (and atomic weights) of nitrogen are found in most nitrogencompounds. The vast majority of chemical reagents, manufactured fertilizers, and environmentalsamples have δ15N values between about −15 and +20 ‰ which corresponds to x(15N) = 0.003 61 to 0.003 74 andAr(N) = 14.006 67 to 14.006 80. Isotope fractionations are caused by physical, chemical, and biologicalprocesses. Some of the largest common effects in the natural environment are caused by microbially mediatedoxidation and reduction reactions and by ammonia or nitric acid evaporation.
The most 15N-enriched occurrences reported in nature include dissolved nitrate that had undergone partial microbialreduction (denitrification) in groundwater (e.g., δ15N = +103 ‰, x(15N) = 0.004 039, andAr(N) = 14.007 10), and nitrate in Antarctic ice that may have been fractionated by evaporation ofHNO3 with δ15N = 150 ‰, x(15N) = 0.004 210, and Ar(N) = 14.007 27.
The most 15N-depleted substances from natural terrestrial environments include nitrous oxide from groundwater undergoing microbial denitrification (δ15N = −55 ‰). Still lower values have been reported forNOx escaping from a nitric acid production facility (δ15N = −150 ‰, x(15N) = 0.003 115, andAr(N) = 14.006 18), and for a commercially available potassium nitrite reagent (δ15N = −80 ‰, x(15N) = 0.003 371, and Ar(N) = 14.006 43).
The annotation 'g' reflects the fact that anumber of samples are known to have atomic weights outside the uncertainties of the standard atomicweight of nitrogen. Many thousands of isotopic analyses of nitrogen have been made since the 1950s; nevertheless,more occurrences of extreme values may be expected as work expands in the fields of contaminant hydrology,biology, and atmospheric chemistry.
Atomic weights of the elements 2009 by M.E. Wieser and T.B. Coplen. Pure Appl. Chem. 2011 (83) 359-396
CIAAW
Nitrogen
Ar(N) = [14.006 43, 14.007 28] since 2009
The name derives from the Latin nitrum and Greek nitron for 'native soda' and genes for 'forming'.Nitrogen was discovered by the Scottish physician and chemist Daniel Rutherford in 1772.
Natural variations of nitrogen isotopic composition
Isotopic reference materials of nitrogen.

Molar mass of N = 14.0067 g/mol
Convert grams Nitrogen to moles or moles Nitrogen to grams
| Symbol | # of Atoms | Nitrogen | N | 14.0067 | 1 | 100.000% |
Nitrogen Atomic Weight Of Gas
Nitrogen Atomic Mass
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Atomic No Of Nitrogen Symbol
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
Atomic No Of Nitrogen Cycle
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
